RUCAM scale to estimate the probability of the drug-induced liver injury (DILI) and herb induced liver injury (HILI) online calculator
The RUCAM score (Roussel Uclaf Causality Assessment Method) helps to assess the likelihood of drug-induced liver injury (DILI), as well as the assessment of the likelihood of herb-induced liver injury (HILI). Previous name CIOMS (Council for International Organizations of Medical Sciences).
Causality assessment by the updated RUCAM requires prior evaluation of liver injury criteria and its pattern in each suspected case
Respective criteria are readily assessable by initial measurement of ALT (alanine aminotransferase) and ALP (alkaline phosphatase).
Updated RUCAM for the Hepatocellular Liver Injury of DILI and HILI.
Drug-induced liver injury is a diagnosis of exclusion, even in cases where the liver injury is likely to be drug-related, the doctor needs to perform a detailed analysis according to certain parameters given in the rating scale.
To assess the causality of drug-induced liver injury (DILI) on the RUCAM scale, it is necessary to select the variant of liver injury (hepatocellular or cholestatic/mixed) and evaluate the indicators corresponding to the variant of liver damage, after the assessment, it is necessary to sum the scale indicators and interpret the scores.
Evaluation of causality using the updated RUCAM requires a preliminary assessment of the criteria for liver injury and its nature in each suspected case.
Appropriate criteria are easily assessed by initially measuring ALT (alanine aminotransferase) and ALP (alkaline phosphatase).
Liver injury is defined by increased serum activities of ALT of at least 5N and/or of ALP of at least 2N, best assessed simultaneously on the day of first presentation (N – upper limit of normal). These thresholds will increase the specificity of the hepatotoxicity causality assessment, eliminates false positive cases, and substantiates hepatotoxicity causality at a high level of probability. They are also in line with a recent consensus on DILI. However, when ALT is within the normal range, ALP increases should be paralleled by increased γ-glutamyltranspeptidase or better 5’ nucleosidase to rule out isolated increases of ALP activities due to bone or another origin rather than hepatobiliary disease
In accordance with the original RUCAM, the updated RUCAM takes into account divergent laboratory constellation of the liver injury and provides two different subscales: one for the hepatocellular type of injury and the other one for the cholestatic or mixed type of injury. These types can be differentiated using the ratio R, calculated as the ALT/ ALP activity measured at the time liver injury is suspected, with both activities expressed as multiples of N.
- The liver injury is hepatocellular if ALT > 5N and ALP ≤ N, or if both ALT and ALP are elevated, R ≥ 5;
- the liver injury is cholestatic if ALP > 2N and ALT ≤ N, or if both ALT and ALP are elevated, R ≤ 2;
- the liver injury is mixed if ALT > 5N and ALP > N and 2 < R < 5.
This classification of liver injury pattern clearly assigns each DILI or HILI case to the updated RUCAM, either for the hepatocellular injury (Table Hepatocellular Liver Injury) or the cholestatic and mixed liver injury (Table Cholestatic or Mixed Liver injury).
Pregnancy is a risk factor only for the cholestatic and mixed liver injury, not for the hepatocellular injury (Table Cholestatic or Mixed Liver injury).
RUCAM scale online calculator
Evaluation of the relationship between liver injyry and taking the drug by the number of points:
Each element of the updated RUCAM is assigned an individual score, and the sum of the individual scores gives the final score for the case. From +14 to -9 points, there is a wide range of final grades.Total score and resulting causality grading:
- ≤0 points – excluded
- 1-2 points – unlikely
- 3-5 points – possible
- 6-8 points – probable
- >9 points – highly probable
What should be considered when using this rating scale?
Operational Information on the Updated RUCAM
- RUCAM affords prospective use, since retrospective scoring is less accurate.
- RUCAM is to be calculated individually for each co-administered product.
- RUCAM is conceptualized primarily for idiosyncratic, not for intrinsic reactions.
- RUCAM excludes cases with onset of hepatic injury before start of product use.
- RUCAM is applicable only for acute liver injury, not for preexisting chronic liver disease.
- RUCAM cannot correctly assess when ALP is elevated for non-hepatic reasons.
- Concomitant use of drugs and herbs is a crucial item that is best inquired and documented at first presentation when liver injury is suspected. Details of a temporal association and potential hepatotoxic features of the used product are to be assessed and documented. For reasons of comparison and transparency, each comedicated drug or herb requires a separate analysis by the complete updated RUCAM. In patients with use of multiple drugs or herbs, the final causality should be attributed primarily to the product with the highest score achieved with the updated RUCAM.
Previous Hepatotoxicity
Hepatotoxicity listed in the product information sheet (e.g., Summary of product characteristics in the EU or product information in the US) must be checked, although the terms used to express the liver injury may vary and usually do not refer to specific definitions. If it is mentioned, then the hepatotoxicity is considered as known for that compound. If this toxicity is not mentioned, a quick literature search in PubMed and the well documented NIH LiverTox website are recommended to determine whether the product has already been involved in published DILI and HILI.
Response to Unintentional Reexposure
A positive reexposure test result is a hallmark and gold standard in DILI cases and recognized by respective scores. To classify a reexposure test as positive, few criteria are required, as specified. The respective criteria were based on the conclusions of International Consensus Meetings in 1988 and 1990, as reviewed previously and recently. For the hepatocellular type of injury, the defining criteria are ALT levels before reexposure (designated as baseline ALT or ALTb), and reexposure ALT levels (designated as ALTr). The reexposure test is positive if ALTb is <5N and ALTr is ≥2ALTb, negative if one or both criteria are not fulfilled, and uninterpretable if data are lacking for one or both criteria. For the cholestatic or the mixed liver injury, the assessment criteria and interpretation of results are similar, with ALT replaced by ALP (Table).
Conditions of unintentional reexposure tests in suspected DILI and HILI cases.
| Reexposure Test Result | Hepatocellular Injury | Cholestatic or Mixed Liver Injury | ||
| ALTb | ALTr | ALPb | ALPr | |
| ● Positive | <5N | ≥2ALTb | <2N | ≥2ALPb |
| ● Negative | <5N | <2ALTb | <2N | <2ALPb |
| ● Negative | ≥5N | ≥2ALTb | ≥2N | ≥2ALPb |
| ● Negative | ≥5N | <2ALTb | ≥2N | <2ALPb |
| ● Uninterpretable | <5N | n.a. | <2N | n.a. |
| ● Uninterpretable | n.a. | ≥2ALTb | n.a. | ≥2ALPb |
| ● Uninterpretable | n.a. | n.a. | n.a. | n.a. |
The original RUCAM was tested for its validity with the use of DILI cases confirmed by positive rechallenge taken as the gold standard and DILI cases where there was strong evidence that drugs were not the culprit drugs. As the updated RUCAM does not change fundamentally the structure and the weights of the original method there is no reason to consider a change in its validity. Presently, all relevant clinical diagnostic parameters are included in the updated RUCAM and in the checklist. There is little evidence that liver histology adds substantially to the diagnosis of DILI or HILI, as these lack specific histopathological features since they mimic all primary hepatic and biliary diseases. Therefore, liver histology is not part of the diagnostic program of the updated RUCAM. However, it is possible to take into account the results of a liver biopsy examination to exclude an alternative cause but certainly not to confirm DILI or HILI.
Search for Alternative Causes
In this domain, the updated RUCAM scale considers the clinically most relevant alternative causes and complications of underlying disease(s). Antibodies are important tools but require assessment and approval by regulatory agencies. Problems of diagnosing infections by hepatitis E virus (HEV) are evident in the US with anti-HEV antibody tests that are not FDA approved. Most importantly, titer changes of antibodies in suspected hepatic viral infections are to be evaluated in the further clinical course to confirm or disprove an ongoing virus infection.
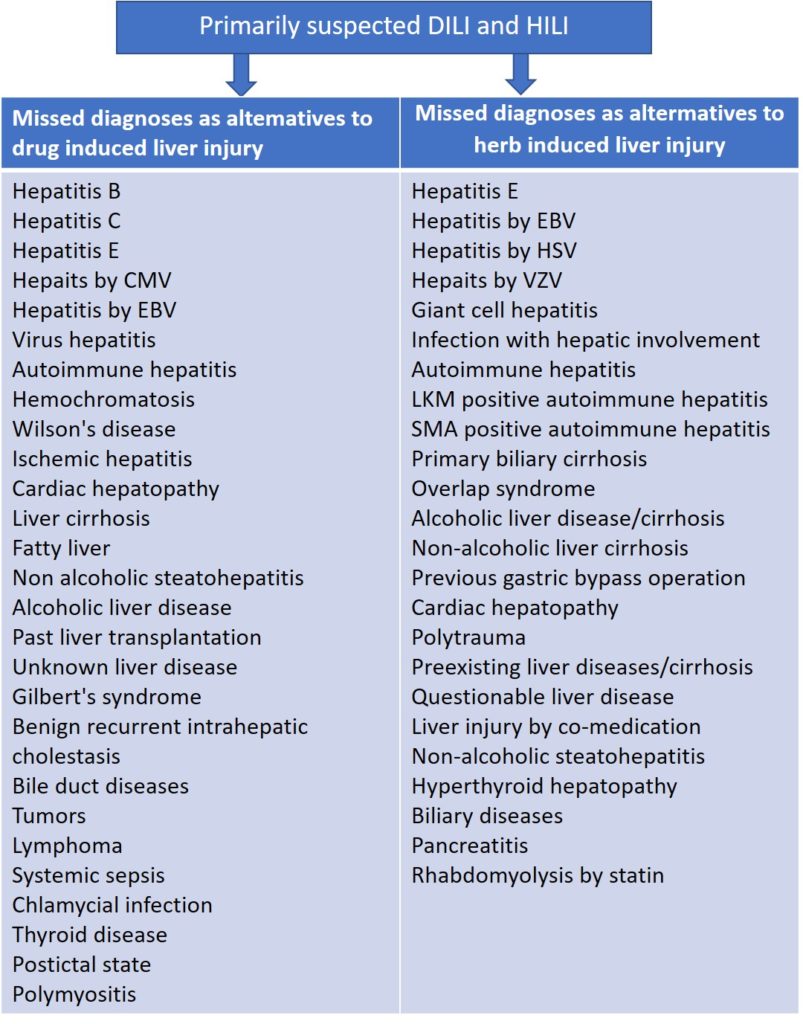
Rare alternative causes are included in a checklist of differential diagnoses as a reminder for the clinician that many diagnoses exist as alternatives to DILI and HILI. These other diagnoses are to be considered, excluded or verified in the context of clinical data and importance, financial resources, and benefit for the patient. Establishing alternative causes contributes to the accuracy of the updated RUCAM and often provides clues to possible specific therapies.
Checklist of differential diagnoses of DILI and HILI
Checklist of differential diagnoses of DILI and HILI. This tabular listing, although not comprehensive, is to be used as a guide and in connection with the updated RUCAM, derived from a previous publication.
| Differential Diagnosis | Diagnostic Parameters | Diagnostic Exclusion Done for Patient’s Assessment | ||
| Yes | No | Partial | ||
| ● Hepatitis A virus (HAV) | Anti-HAV-IgM | □ | □ | □ |
| ● Hepatitis B virus (HBV) | HBV-DNA, anti-HBc-IgM | □ | □ | □ |
| ● Hepatitis C virus (HCV) | HCV-RNA, anti-HCV | □ | □ | □ |
| ● Hepatitis E virus (HEV) | HEV-RNA, titer change for anti-HEV-IgM/anti-HEV-IgG | □ | □ | □ |
| ● Cytomegalovirus (CMV) | CMV-PCR, titer change for anti-CMV-IgM/anti-CMV-IgG | □ | □ | □ |
| ● Epstein Barr virus (EBV) | EBV-PCR, titer change for anti-EBV-IgM/anti-EBV-IgG | □ | □ | □ |
| ● Herpes simplex virus (HSV) | HSV-PCR, titer change for anti-HSV-IgM/anti-HSV-IgG | □ | □ | □ |
| ● Varicella zoster virus (VZV) | VZV-PCR, titer change for anti-VZV-IgM/anti-VZV-IgG | □ | □ | □ |
| ● Other virus infections | Specific serology of Adenovirus, Coxsackie-B-Virus, Echovirus, Measles virus, Rubella virus, Flavivirus, Arenavirus, Filovirus, Parvovirus, HIV, and others | □ | □ | □ |
| ● Other infectious diseases | Specific assessment of bacteria, fungi, parasites, worms, and others | □ | □ | □ |
| ● Autoimmune hepatitis (AIH) type I | Gamma globulins, ANA, SMA, AAA, SLA/LP, Anti-LSP, Anti-ASGPR | □ | □ | □ |
| ● Autoimmune hepatitis (AIH) type II | Gamma globulins, Anti-LKM-1 (CYP 2D6), Anti-LKM-2 (CYP 2C9), Anti-LKM-3 | □ | □ | □ |
| ● Primary biliary cholangitis (PBC) | AMA, Anti PDH-E2 | □ | □ | □ |
| ● Primary sclerosing cholangitis (PSC) | p-ANCA, MRC | □ | □ | □ |
| ● Autoimmune cholangitis (AIC) | ANA, SMA | □ | □ | □ |
| ● Overlap syndromes | See AIH, PBC, PSC, and AIC | □ | □ | □ |
| ● Non alcoholic steatohepatitis (NASH) | BMI, insulin resistance, hepatomegaly, echogenicity of the liver | □ | □ | □ |
| ● Alcoholic liver disease (ALD) | Patient’s history, clinical and laboratory assessment, other alcoholic disease(s) | □ | □ | □ |
| ● Drug induced liver injury (DILI) or herb induced liver injury (HILI) | Patient’s history, clinical and laboratory assessment, sonography, use of the updated RUCAM | □ | □ | □ |
| ● Cocaine, ecstasy and other amphetamines | Toxin screening | □ | □ | □ |
| ● Rare intoxications | Toxin screening for household and occupational toxins | □ | □ | □ |
| ● Hereditary hemochromatosis | Serum ferritin, total iron-binding capacity, genotyping for C2824 and H63D mutation, hepatic iron content | □ | □ | □ |
| ● Wilson disease | Copper excretion (24 h urine), ceruloplasmin in serum, free copper in serum, Coombs-negative hemolytic anemia, hepatic copper content, Kayser-Fleischer-ring, neurologic-psychiatric work-up, genotyping | □ | □ | □ |
| ● Porphyria | Porphobilinogen in urine, total porphyrines in urine | □ | □ | □ |
| ● α1—Antitrypsin deficiency | α1—Antitrypsin in serum | □ | □ | □ |
| ● Biliary diseases | Clinical and laboratory assessment, hepatobiliary sonography, MRC | □ | □ | □ |
| ● Pancreatic diseases | Clinical and laboratory assessment, sonography, CT, MRT | □ | □ | □ |
| ● Celiac disease | TTG antibodies, endomysium antibodies, duodenal biopsy | □ | □ | □ |
| ● Anorexia nervosa | Clinical context | □ | □ | □ |
| ● Parenteral nutrition | Clinical context | □ | □ | □ |
| ● Cardiopulmonary diseases | Cardiopulmonary assessment of congestive heart disease, myocardial infarction, cardiomyopathy, cardiac valvular dysfunction, pulmonary embolism, pericardial diseases, arrhythmia, hemorrhagic shock, and various other conditions | □ | □ | □ |
| ● Addison’s disease | Plasma cortisol | □ | □ | □ |
| ● Thyroid diseases | TSH basal, T4, T3 | □ | □ | □ |
| ● Grand mal seizures | Clinical context of epileptic seizure (duration > 30 min) | □ | □ | □ |
| ● Heat stroke | Shock, hyperthermia | □ | □ | □ |
| ● Polytrauma | Shock, liver injury | □ | □ | □ |
| ● Systemic diseases | Specific assessment of sarcoidosis, amyloidosis, metastatic tumor, sepsis, and others | □ | □ | □ |
| ● Other diseases | Clinical context | □ | □ | □ |
Thus, the updated RUCAM can be used based on:
- Type of the damage to the liver (hepatocellular, cholestatic, mixed type);
- Detailing of the main elements of assessments (table hepatocellular, cholestatic/mixed type of damage);
- Differential diagnosis (checklist table)
- Reexposure criteria (table).
Correct Diagnoses
Results based on the updated RUCAM are quickly available for trained physicians with substantial clinical experience who then have to decide whether DILI or HILI is the most like diagnosis in their patients or any other differential diagnoses. Their decision is crucial, as DILI and HILI require discontinuation of the offending product whereas other diagnoses may require specific therapeutic modalities. The patient will immediately profit from the correct diagnosis achieved after using the updated RUCAM and the checklist.
Missed Diagnoses
Unrecognized alternative causes to the liver injury are a real clinical problem when caring for patients with initially assumed but later not confirmed DILI or HILI; the long list of missed diagnoses in the setting of initially assumed injury cases is indeed threatening. More recent evidence suggests that the problem of missed diagnoses is multifaceted and caused by incomplete case data collection, poor case data analysis, problems of appropriate case data transfer from medical files to the manuscript, and unjustified upgrading of causality scores. Missing the correct diagnosis may cause legal considerations that are better avoided by prior sophisticated clinical and regulatory approaches. Effective treatments would have been available for some patients with missed diagnoses, a critical situation in the clinical context.
Abbreviations: CMV, Cytomegalovirus; EBV, Epstein Barr virus; HSV Herpes simplex virus; LKM, Liver kidney microsomes; SMA, Smooth muscle antibodies; VZV, Varicella zoster virus.
Missed diagnoses in cases of initially suspected hepatotoxicity by synthetic drugs or herbs. Adapted from previous reports, which provide the respective references for each missed diagnosis listed above.
Historical background
Previous synonym of RUCAM were CIOMS (Council for International Organizations of Medical Sciences) or CIOMS/RUCCAM.
The updated RUCAM is as good as physicians and assessors are handling this method and consider basic liver tests for the liver injury classification, the specific operational information, details of core elements and their scoring, differential diagnoses, and the reexposure criteria. RUCAM has not been designed for chronic DILI and HILI or when a suspected injury occurs on pre-existing liver disease, both complex conditions where an expert panel of hepatologists would provide a more accurate approach especially for the timing of the events and the exclusion of alternative causes.
Historical background and the original work confirm the use of RUCAM as single term for future cases, dismissing now the term CIOMS for reasons of simplicity and clarity. RUCAM represents a structured, standardized, validated, and hepatotoxicity specific diagnostic approach that attributes scores to individual key items, providing final quantitative gradings of causality for each suspect drug/herb in a case report.
Experts from Europe and the United States had previously established in consensus meetings the first criteria of RUCAM to meet the requirements of clinicians and practitioners in care for their patients with suspected DILI and HILI. RUCAM was completed by additional criteria and validated, assisting to establish the timely diagnosis with a high degree of certainty. In many countries and for more than two decades, physicians, regulatory agencies, case report authors, and pharmaceutical companies successfully applied RUCAM for suspected DILI and HILI. Their practical experience, emerging new data on DILI and HILI characteristics, and few ambiguous questions in domains such alcohol use and exclusions of non-drug causes led to the present update of RUCAM. The aim was to reduce interobserver and intraobserver variability, to provide accurately defined, objective core elements, and to simplify the handling of the items. The authors presented the update of the well accepted original RUCAM scale and recommend its use for clinical, regulatory, publication, and expert purposes to validly establish causality in cases of suspected DILI and HILI, facilitating a straightforward application and an internationally harmonized approach of causality assessment as a common basic tool.
RUCAM was not designed for chronic DILI and HILI, or when damage is suspected in pre-existing liver disease.
RUCAM scoring can help establish a clinical diagnosis, which can be useful for further choice of therapy.
Register on our website right now to have access to more learning materials!
Subscribe to our pages:
Literature
- Danan G., Bénichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [PubMed]
- Andrade RJ, Robles M, Fernández-Castañer A, López-Ortega S, López-Vega MC, Lucena MI (2007).
“Assessment of drug-induced hepatotoxicity in clinical practice: a challenge for gastroenterologists.” World J. Gastroenterol. 13 (3): 329–40. - Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci. 2015;17(1):14. Published 2015 Dec 24. doi:10.3390/ijms17010014
- Teschke R., Eickhoff A., Schwarzenboeck A., Schmidt-Taenzer W., Genthner A., Frenzel C., Wolff A., Schulze J. Clinical review: Herbal hepatotoxicity and the call for systematic data documentation of individual cases. J. Liver Clin. Res. 2015;2:1008. (Google Scholar)
See also:
MELD Score (Model For End-Stage Liver Disease) (12 and older): Online calculator
MELD Score stratifies severity of end-stage liver disease, for transplant planning. NB! MELD Score can…
Interactive Checklist of differential diagnoses of DILI and HILI
Differential diagnoses of drug-induced and herb-induced liver disease: Checklist of differential diagnoses of DILI and…
RUCAM scale to estimate the probability of the drug-induced liver injury (DILI) and herb induced liver injury (HILI) online calculator
The RUCAM score (Roussel Uclaf Causality Assessment Method) helps to assess the likelihood of drug-induced…
R-value for Liver Injury Online calculator
Can be used in patients with suspected drug induced liver injury (DILI) with abnormal liver…
Child-Pugh Score for Cirrhosis Mortality: online calculator
The Child-Pugh Score can be useful in the prognosis of patients with cirrhosis, but more…
King’s College Criteria for Non- Acetaminophen Toxicity acute hepatic failure
Identification of patients who should be immediately referred for liver transplant. INR > 6.5 (Prothrombin…