Two masks of the same syndrome: Wellens syndrome

Early reperfusion therapy is a gold standard strategy in the management of patients with acute ST-segment elevation coronary syndrome (STEMI-ACS), as elevation on the electrocardiogram (ECG) is recognized as a manifestation of critical stenosis or occlusion of the coronary artery.
At the same time, about 10 – 25% of patients with an acute coronary syndrome without ST-segment elevation, according to coronary angiography, also have critical stenosis or occlusion of a large epicardial artery. A delay in revascularization in this group of patients is associated with worse outcomes. Although the current guidelines briefly mention the criteria for STEMI ACS equivalents, there is still insufficient information on specific ECG patterns.
In this publication, we will consider an electrocardiographic phenomenon – Wellens syndrome.
Introduction
Wellens Syndrome is a clinical syndrome characterised by biphasic or deeply inverted T waves in V2-3, plus a history of recent chest pain now resolved. It is represent highly specific for critical stenosis of the left anterior descending artery (LAD).
Wellens syndrome was firstly described in the early 1980s by de Zwaan, Wellens, and colleagues, who identified a subset of patients with unstable angina who had specific precordial T-wave changes and subsequently developed a large anterior wall myocardial infarction (MI). Wellens syndrome refers to these specific electrocardiographic (ECG) abnormalities in the precordial T-wave segment, which are associated with critical stenosis of the proximal left anterior descending (LAD) coronary artery.
Wellens syndrome is also referred to as LAD coronary T-wave syndrome. Syndrome criteria include the following:
- Characteristic T-wave changes
- History of anginal chest pain and absence of pain syndrome during identification typical changes on the ECG
- Normal or minimally elevated cardiac enzyme levels
- ECG without Q waves, without significant ST-segment elevation, and with normal precordial R-wave progression
Recognition of this ECG abnormality has great significance under this syndrome represents a preinfarction stage of coronary artery disease (CAD) that often progresses to a devastating anterior wall MI.
Pathophysiology
Wellens syndrome represents critical stenosis of the proximal segment of the LAD artery. The LAD arises from the left coronary artery and passes through the interventricular groove along the anterior portion of the heart to the apex. This groove is situated between the right and left ventricles of the heart. The LAD gives rise to 2 main branches, the diagonals, and the septal perforators.
A lesion in the proximal LAD can have severe consequences, as suggested by the common title given to this lesion: “widow maker.” The LAD supplies the anterior wall of the heart, including both ventricles, as well as the septum. An occlusion in this vessel can result in serious ventricular dysfunction, thus placing the patient at serious risk for congestive heart failure (CHF) and death.
Etiology
Wellens syndrome is a preinfarction stage of CAD. The causes of Wellens syndrome are similar to the conditions that cause CAD, including the following:
- Atherosclerotic plaque
- Coronary artery vasospasm (cocaine is one possible cause)
- Increased cardiac demand
- Generalized hypoxia
Risk factors for Wellens syndrome are essentially those of CAD and include the following:
- Smoking history
- Diabetes mellitus
- Hypertension
- Increased age
- Hypercholesterolemia
- Hyperlipidemia
- Metabolic syndrome
- Family history of premature heart disease
- Occupational stress
Epidemiology and Prognosis
The characteristic ECG pattern of Wellens syndrome is relatively common in patients who have symptoms consistent with unstable angina. Among patients admitted with unstable angina, this ECG pattern is present in 14-18% cases.
Wellens syndrome represents critical proximal segment LAD disease; accordingly, its natural progression leads to anterior wall MI. This progression is so likely that medical management alone is not enough to stop the natural process. Evolution to an anterior wall MI is rapid, with a mean time of 8.5 days from the onset of Wellens syndrome to infarction.
If anterior wall MI occurs, there is the potential for substantial morbidity or mortality. Thus, it is of utmost importance to recognize this pattern early.
Clinical Presentation
Wellens syndrome represents stenosis of the proximal segment of the left anterior descending coronary artery (LAD), and patients typically present with symptoms or complaints consistent with coronary artery disease (CAD). Generally, the history is most consistent with unstable angina. Angina can have varying presentations, but the classic presentation includes the following complaints:
- Chest pain described as pressure, tightness, or heaviness
- Pain that is typically induced by activity and relieved by rest
- Radiation of pain to the jaw, shoulder, or neck
May experience multiple associated symptoms, including diaphoresis, nausea, vomiting, and fatigue.
Elderly, diabetic, and female patients are more likely to present with atypical symptoms.
Physical Examination
Physical examination does not provide any indicators that would give the strong grounds for suspecting Wellens syndrome specifically. However, the results of the patient’s examination may show evidence of ongoing ischemic injury (congestive heart failure).
In addition, most of the electrocardiographic (ECG) changes are recognized when the patient is pain-free, which again underscores the importance of a repeat pain-free ECG in the emergency department.
Diagnostic strategy
Potential diagnostic pitfalls with Wellens syndrome include the following:
- Failing to recognize Wellens syndrome T-wave changes on electrocardiography
- Ordering a stress test without recognizing risks (see Workup); this may provoke a large anterior wall myocardial infarction (MI)
- Underestimating the seriousness of the ECG finding in a pain-free patient
- Failing to properly admit or consult cardiology on patients with the characteristic ECG changes
Differential Diagnoses
- Acute Coronary Syndromes
- Acute Pericarditis
- Angina Pectoris
- Atherosclerosis
- Bacterial Pneumonia
- Coronary Artery Atherosclerosis
- Myocardial Infarction
- Myocarditis
- Pulmonary Embolism (PE)
In addition to the conditions listed in the differential diagnosis, other conditions to be considered include the following:
- Central nervous system injury
- Persistent juvenile T-wave pattern
- Left ventricular hypertrophy
- Bundle-branch block
- Digitalis effect
- Acute myocarditis
- Preexcitation syndromes
- Later stages of pericarditis
Laboratory tests
The following laboratory studies may be indicated as adjunctive tests in patients with suspected coronary artery disease (CAD), acute coronary syndrome (ACS), and Wellens syndrome:
- Complete blood count (CBC) – To ensure that anemia is not precipitating the angina, red blood cell (RBC) transfusions may be necessary
- Basic metabolic profile – This includes electrolyte, blood urea nitrogen (BUN), creatinine, and glucose levels
- D-dimer, international normalized ratio (INR), partial thromboplastin time (PTT) – These are ordered only as medically necessary
- Cardiac biomarkers – In Wellens syndrome, cardiac biomarkers can be falsely reassuring, in that they are typically normal or only minimally elevated; only 12% of patients with this syndrome have elevated cardiac biomarker levels, and these are always less than twice the upper limit of normal, in the absence of myocardial infarction (MI)
Radiography, CT of Chest
Chest radiography should be performed to look for side effects of ischemia, such as pulmonary edema. In addition, this test should be performed to help exclude other possible causes of chest pain, such as thoracic aneurysm or dissection, pneumonia, and rib fracture.
Computed tomography (CT) of the chest is performed only as indicated to help rule out other causes of chest pain, such as aortic dissection or pulmonary embolus. The value of CT angiography of the chest in the evaluation of chest pain, CAD, and ACS is currently being investigated.
Electrocardiography
ECG should be performed on any patient with a complaint of chest pain if noncardiac causes cannot be diagnosed by other means, including physical examination. It may be helpful to perform ECG both while the pain is present and after the pain has resolved. The ECG changes seen in Wellens syndrome typically are manifested when the patient is pain-free but usually occur in the context of recent anginal chest pain.
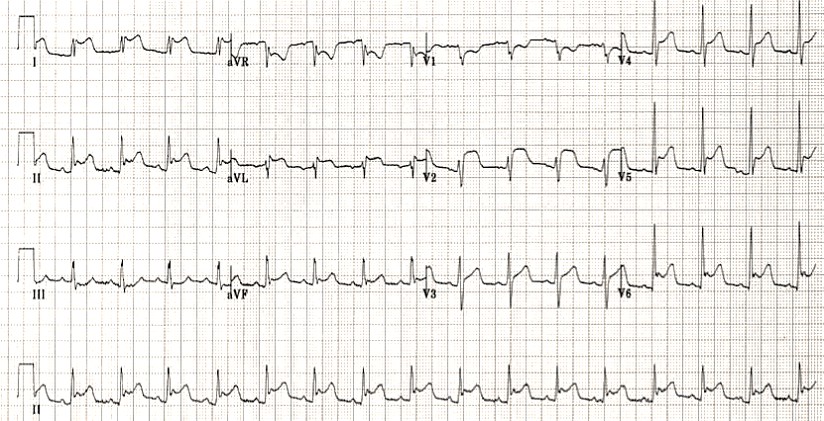
In Wellens syndrome, the ECG pattern shows significant involvement of the T wave, with minimal ST-segment alteration. The ST segments themselves are usually isoelectric, but if they are abnormal, there will be less than 1 mm of elevation, with a high takeoff of the ST segment from the QRS complex. The characteristic ECG changes of this syndrome occur in the T-wave and occur in 2 forms.
There are two patterns of T-wave abnormality in Wellens syndrome:
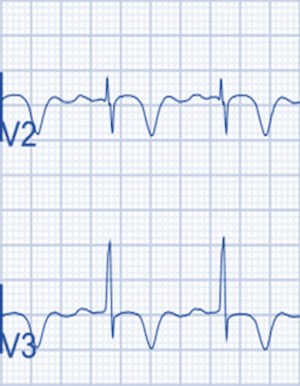
- Type A – Biphasic, with initial positivity and terminal negativity (24% of cases)
- Type B – Deeply and symmetrically inverted (76% of cases)
Biphasic T Waves (Type A)
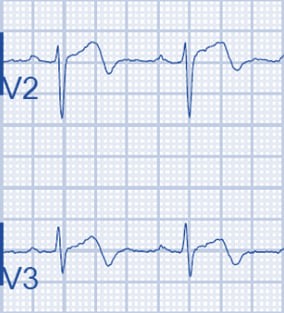
The more common of the 2 forms, which occurs 76% of the time, is deep inversion of the T-wave segment in the precordial leads (see the images below). The ST segment will be straight or concave and will pass into a deep negative T wave at an angle of 60°-90°. The T wave is symmetric. In Wellens syndrome, these changes generally occur in leads V1 -V4 but occasionally may also involve V5 and V6. V1 is involved in approximately 66% of patients, and V4 is involved nearly 75% of the time.
Deeply Inverted T Waves (Type B)
The less common of the 2 forms of Wellens syndrome, which occurs in 24% of patients, consists of biphasic T waves (see the images below), most commonly in leads V2 and V3 but also, on occasion, in leads V1 through V5 or even V6. The characteristic pattern classically presents only during chest pain–free periods. Noticing this pattern is crucial because it is a sign of LAD disease. This association underscores the importance of serial ECGs and a pain-free ECG on patients with unstable angina. Because the LAD supplies the anterior myocardium, failure to recognize this pattern can result in anterior wall myocardial infarction (MI), significant left ventricular dysfunction, or death.
Reperfusion of the posterior wall following posterior MI leads to a larger T-wave amplitude in the right precordial leads and an even higher T-wave amplitude in leads V2 and V3.
Wellens T wave evolution
T wave changes can evolve over time from Type A to Type B pattern (Smith et al).
Evolution of T-wave inversion [A-D] after coronary reperfusion in STEMI reperfusion and in Wellens syndrome (NSTEMI). Modified from Smith et al. Evolution of T-wave inversion. The ECG in acute MI, 2002
Diagnostic criteria
Rhinehart et al (2002) describe the following diagnostic criteria for Wellens syndrome:
- Deeply inverted or biphasic T waves in V2-3 (may extend to V1-6)
- ECG pattern present in pain-free state
- Isoelectric or minimally-elevated ST segment (< 1mm)
- No precordial Q waves
- Preserved precordial R wave progression
- Recent history of angina
- Normal or slightly elevated serum cardiac markers
Understanding T wave changes
The following sequence of events is thought to occur in patients with Wellens syndrome:
- A sudden complete occlusion of the LAD causes a transient anterior STEMI, causing chest pain & diaphoresis. This stage may not be successfully captured on an ECG recording
- Re-perfusion of the LAD (e.g. due to spontaneous clot lysis or prehospital aspirin) leads to resolution of chest pain. ST elevation improves and T waves become biphasic or inverted. The T wave morphology is identical to patients who reperfuse after a successful PCI
- If the artery remains open, the T waves evolve over time from biphasic to deeply inverted
- The coronary perfusion is unstable, however, and the LAD can re-occlude at any time. If this happens, the first sign on the ECG is an apparent normalisation of the T waves — so-called “pseudo-normalisation”. The T waves switch from biphasic/inverted to upright and prominent. This is a sign of hyperacute STEMI and is usually accompanied by recurrence of chest pain, although the ECG changes can precede the symptoms
- If the artery remains occluded, the patient now develops an evolving anterior STEMI
- Alternatively, a “stuttering” pattern may develop, with intermittent reperfusion and re-occlusion. This would manifest as alternating ECGs demonstrating Wellens and pseudonormalisation/STEMI patterns
- This sequence of events is not limited to the anterior leads — similar changes may be seen in the inferior or lateral leads, e.g. with RCA or circumflex occlusion.
Also, the inciting event does not necessarily have to be thrombus formation — Wellens syndrome may also occur in normal coronary arteries following an episode of vasospasm, as in this case of cocaine-induced vasospasm. However, it is safer to assume the worst (i.e. critical LAD stenosis) and work the patient up for an angiogram.
Other Tests
Angiography has demonstrated that 100% of patients with Wellens syndrome will have 50% or greater stenosis of the proximal LAD. More specifically, 83% will have the lesion proximal to the second septal perforator.
Stress testing is generally not indicated in patients with this ECG presentation, because it places them at risk for acute anterior wall MI. Ideally, therefore, these patients would bypass stress testing and undergo urgent angiography to determine the extent of disease and potentially provide information about the need for percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), or medical management. If provocative testing is determined to be necessary, it should be done cautiously and only in close conjunction with a cardiologist.
Treatment Approach
A cardiologist should be consulted as early as possible in the management of a patient with Wellens syndrome. If the patient remains pain-free, it is appropriate to admit him or her to an internist on a telemetry floor, but the internist should be notified that the patient is at high risk and should not undergo stress testing unless clearly recommended by cardiologists.
If symptoms persist or electrocardiography shows evolution into ST-segment elevations, an interventional cardiologist should be consulted immediately. Transfer of these patients to institutions with cardiac catheterization capabilities is generally appropriate.
First aid (prehospital stage)
Because Wellens syndrome occurs because of stenosis of the proximal left anterior descending coronary artery, patients typically complain of chest pain presenting as unstable angina. During episodes of pain, they should be treated in the same manner as any patient experiencing chest pain thought to be cardiac in origin.
Immediate arrangements should be made for transport to the nearest hospital. Careful attention should be paid to the ABCs (airway, breathing, and circulation). During transport, efforts should be made to carry out the following:
- Oxygen supplementation
- Assessment of vital signs
- Intravenous (IV) access
- Administration of aspirin
- ECG, if available before arrival at the hospital
- If pain persists, administration of nitroglycerin or morphine, according to local protocols
If Wellens syndrome is identified on an outpatient basis, then arrangements should be made for urgent evaluation. Stress testing should be avoided.
Medical care during hospital stage
Patients presenting with symptoms consistent with unstable angina should generally receive medications and other therapies and measures that may help prevent myocardial infarction (MI). Usually, these would include the following:
- IV access
- Supplemental oxygen, when appropriate
- ECG (initially) – Serial examinations and pain-free tracings may be helpful
- Telemetry monitoring
- Chest radiography
- Laboratory studies (see Laboratory Studies)
- Aspirin
- Consideration should be given to providing beta-blocker therapy, nitroglycerin, morphine, heparin, clopidogrel, and glycoprotein (GP) IIb/IIa inhibitors
Once again, the ECG changes in Wellens syndrome are typically only present when the patient is free of chest pain. Thus, obtaining serial ECGs on patients with unstable angina may be helpful.
Even though the ECG changes may be subtle, it is vital to recognize Wellens syndrome because these patients can rarely undergo stress testing safely. Because Wellens syndrome is a sign of a preinfarction stenosis of the LAD, a stress test has the potential to result in acute MI and severe damage to the left ventricle. Therefore, these patients should generally forgo a stress test and instead may undergo angiography to evaluate the need for angioplasty or coronary artery bypass surgery (CABG).
Even with ideal medical management, the natural progression of Wellens syndrome is to acute anterior wall MI. Approximately 75% of patients with Wellens syndrome who receive only medical management and do not undergo revascularization (either through CABG or through angioplasty) will go on to develop extensive anterior wall MI within days. Anterior wall MI carries substantial morbidity and mortality: it will result in left ventricular dysfunction and possibly even death.
Thus, patients generally should be medically stabilized if possible while arrangements are made for urgent angiography and revascularization if appropriate.
The goals of pharmacotherapy are to reduce morbidity and prevent complications. Agents used in the management of Wellens syndrome include salicylates, antihypertensive drugs, antianginal drugs, antiplatelet drugs, analgesics, low-molecular-weight heparins (LMWHs), antiarrhythmic drugs, and anticoagulants.
Register on our website right now to have access to more learning materials!
References
- Mike Cadogan and Robert Buttner Wellens Syndrome. Ecg library. Sep 2021 https://litfl.com/wellens-syndrome-ecg-library/
- de Zwaan C, Bar FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982 Apr. 103(4 Pt 2):730-6. [Medline].
- Nisbet BC, Zlupko G. Repeat Wellens’ syndrome: case report of critical proximal left anterior descending artery restenosis. J Emerg Med. 2010 Sep. 39(3):305-8. [Medline].
- Patel K, Alattar F, Koneru J, Shamoon F. ST-elevation myocardial infarction after pharmacologic Persantine stress test in a patient with Wellens’ syndrome. Case Rep Emerg Med. 2014. 2014:530451. [Medline]. [Full Text].
- Moore KL, Dalley AF. Thorax. Clinically Oriented Anatomy. 4th ed. Baltimore, Maryland: Lippincott Williams & Wilkins; 1999. 135.
- de Zwaan C, Bar FW, Janssen JH, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J. 1989 Mar. 117(3):657-65. [Medline].
- Tandy TK, Bottomy DP, Lewis JG. Wellens’ syndrome. Ann Emerg Med. 1999 Mar. 33(3):347-51. [Medline].
- Rhinehardt J, Brady WJ, Perron AD, Mattu A. Electrocardiographic manifestations of Wellens’ syndrome. Am J Emerg Med. 2002 Nov. 20(7):638-43. [Medline].
- Driver BE, Shroff GR, Smith SW. Posterior reperfusion T-waves: Wellens’ syndrome of the posterior wall. Emerg Med J. 2017 Feb. 34(2):119-23. [Medline].
- Tatli E, Aktoz M, Buyuklu M, Altun A. Wellens’ syndrome: the electrocardiographic finding that is seen as unimportant. Cardiol J. 2009. 16(1):73-5. [Medline].
- Narasimhan S, Robinson GM. Wellens syndrome: a combined variant. J Postgrad Med. 2004 Jan-Mar. 50(1):73-4. [Medline].
- Sowers N. Harbinger of infarction: Wellens syndrome electrocardiographic abnormalities in the emergency department. Can Fam Physician. 2013 Apr. 59(4):365-6. [Medline]. [Full Text].
- Balasubramanian K, Balasubramanian R, Subramanian A. A dangerous twist of the ‘T’ wave: A case of Wellens’ Syndrome. Australas Med J. 2013. 6(3):122-5. [Medline]. [Full Text].
- Singh B, Singh Y, Singla V, Nanjappa MC. Wellens’ syndrome: a classical electrocardiographic sign of impending myocardial infarction. BMJ Case Rep. 2013 Feb 18. 2013:[Medline].
- Movahed MR. Wellens’ syndrome or inverted U-waves?. Clin Cardiol. 2008 Mar. 31(3):133-4. [Medline].
- Y-Hassan S. The pathogenesis of reversible T-wave inversions or large upright peaked T-waves: Sympathetic T-waves. Int J Cardiol. 2015 Jul 15. 191:237-43. [Medline].
- Lin AN, Lin S, Gokhroo R, Misra D. Cocaine-induced pseudo-Wellens’ syndrome: a Wellens’ phenocopy. BMJ Case Rep. 2017 Dec 14. 2017:[Medline].
- Abuarqoub A, Naranjo M, Shamoon F. Myocardial bridging with left ventricular hypertrophy presenting as Wellens pattern. Ann Transl Med. 2017 Oct. 5(20):401. [Medline]. [Full Text].
- Hsu YC, Hsu CW, Chen TC. Type B Wellens’ syndrome: Electrocardiogram patterns that clinicians should be aware of. Ci Ji Yi Xue Za Zhi. 2017 Apr-Jun. 29(2):127-8. [Medline]. [Full Text].
Baseline Cardiovascular Risk Assessment in Cancer Patients Scheduled to Receive Cardiotoxic Cancer Therapies (Anthracycline Chemotherapy) – Online Calculator
Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies (Anthracycline Chemotherapy)…
SAVED VTE Score
SAVED score for venous thromboembolism risk stratification in patients with multiple myeloma receiving immunomodulators. [ezfc…
IMPEDE VTE Score
IMPEDE score for venous thromboembolism risk stratification in patients with multiple myeloma receiving immunomodulators. [ezfc…
Chest CT Scan
To analyze a CT scan of the chest, imagine that the patient is lying on…
X-ray Heart Borders
According to the radiograph of the chest, the boundaries of the heart are formed: The…
Emergency care of a patient with chest pain – Acute Coronary Syndrome with ST-segment elevation and equivalents /OSCE guide
Opening the consultationWash your hands and don PPE if appropriateIntroduce yourself to the patient including…